
SL Paper 3
of the mass of a rock weighing is uranium(IV) oxide, . of the uranium atoms in the rock are uranium-238, .
Show that the mass of the isotope in the rock is .
The half-life of 238U is years. Calculate the mass of 238U that remains after has decayed for years.
Outline a health risk produced by exposure to radioactive decay.
Deduce the nuclear equation for the decay of uranium-238 to thorium-234.
Thorium-234 has a higher binding energy per nucleon than uranium-238. Outline what is meant by the binding energy of a nucleus.
Markscheme
// ✔
✔
Award [1 max] for omitting mass composition (giving ).
M2 is for numerical setup, not for final value of .
Alternative 1
✔
✔
Alternative 2
✔
✔
Award [2] for correct final answer.
Any one:
«genetic» mutations ✔
«could cause» cancer ✔
Accept specific named types of cancer.
cells «in body» altered ✔
cells «in body» cannot function ✔
damaged DNA/proteins/enzymes/organs/tissue ✔
«radiation» burns ✔
hair loss ✔
damage in foetuses ✔
damages/weakens immune system ✔
✔
Do not penalize missing atomic numbers in the equation.
Accept “” for "”.
energy required to separate a nucleus into protons and neutrons/nucleons
OR
energy released when nucleus was formed from «individual/free/isolated» protons and neutrons/nucleons ✔
Do not accept “energy released when atom was formed”.
Examiners report
This was a very different question as student were given the answer and it was the work that was being marked. Students should always clearly show their calculations so examiners can award marks throughout the question and potentially award ECF if possible. It is very difficult to do this when students do not show work clearly.
This continues to be a topic that students understand well, and probably more used alternative 1 to calculate the half-life. Students should always clearly show their calculations so examiners can award marks throughout the question and potentially award ECF if possible. It is very difficult to do this when students do not show work clearly.
A well answered question.
Only very weak candidates lost this mark.
Many students appeared to have portion of the answer but not the entire concept. They seemed to have studied previous MS by heart and entered an answer that was mostly correct but didn't address the question. It is important that student understand the material and not try to memorize their way through the topics.
The mild analgesic aspirin can be prepared in the laboratory from salicylic acid.
(CH3CO)2O + HOC6H4COOH → CH3CO2C6H4COOH + CH3COOH
Salicylic acid Aspirin
After the reaction is complete, the product is isolated, recrystallized, tested for purity and the experimental yield is measured. A student’s results in a single trial are as follows.
Literature melting point data: aspirin = 138–140 °C
Determine the percentage experimental yield of the product after recrystallization. The molar masses are as follows: M(salicylic acid) = 138.13 g mol−1, M(aspirin) = 180.17 g mol−1. (You do not need to process the uncertainties in the calculation.)
Suggest why isolation of the crude product involved the addition of ice-cold water.
Justify the conclusion that recrystallization increased the purity of the product, by reference to two differences between the melting point data of the crude and recrystallized products.
State why aspirin is described as a mild analgesic with reference to its site of action.
Markscheme
ALTERNATIVE 1:
«theoretical yield = × 180.17 g mol−1 =» 2.024 «g»
«experimental yield = × 100 =» 55.53 «%»
ALTERNATIVE 2:
«»= 0.01124 «mol salicylic acid/aspirin theoretical» AND
«»= 0.006239 «mol aspirin experimental»
«experimental yield = x 100 =» 55.51 «%»
Accept answers in the range 55.4 % to 55.7 %.
Award [2] for correct final answer.
low temperature gives greater difference between solubility of aspirin and impurities
OR
«product» crystallizes out from cold solution/«ice-cold water/lower temperature» speeds up crystallization process
OR
aspirin/product has low solubility «in water» at low temperatures
intercepts pain stimulus at source/acts at site of pain
OR
interferes with production of pain sensitizing substances/prostaglandins «at site of pain»
Examiners report
recrystallized melting point is higher
OR
recrystallized melting point is closer to pure substance/literature value
smaller range of values
An investigation was carried out to determine the effect of chain length of the alcohol on the equilibrium constant, , for the reversible reaction:
The reactants, products and the catalyst form a homogeneous mixture.
Fixed volumes of each alcohol, the ethanoic acid and the sulfuric acid catalyst were placed in sealed conical flasks.
At equilibrium, the flasks were placed in an ice bath, and samples of each flask titrated with to determine the ethanoic acid concentration present in the equilibrium mixture.
The following processed results were obtained.
© International Baccalaureate Organization 2020
Identify the independent and dependent variables in this experiment.
The ice bath is used at equilibrium to slow down the forward and reverse reactions. Explain why adding a large amount of water to the reaction mixture would also slow down both reactions.
Suggest why the titration must be conducted quickly even though a low temperature is maintained.
An additional experiment was conducted in which only the sulfuric acid catalyst was titrated with . Outline why this experiment was necessary.
Calculate the percentage uncertainty and percentage error in the experimentally determined value of for methanol.
Comment on the magnitudes of random and systematic errors in this experiment using the answers in (e).
Suggest a risk of using sulfuric acid as the catalyst.
Markscheme
Independent variable:
chain length OR number of carbon «atoms in alcohol»
AND
Dependent variable:
volume of OR /equilibrium constant OR equilibrium concentration/moles of ✔
dilution/lower concentrations ✔
less frequent collisions «per unit volume» ✔
Accept “lowers concentration of acid catalyst” for M1. M2 must refer to increase in activation energy or different pathway.
Do not accept responses referring to equilibrium.
equilibrium shifts to left
OR
more ethanoic acid is produced «as ethanoic acid is neutralized»
OR
prevents/slows down ester hydrolysis ✔
Accept “prevents equilibrium shift” if described correctly without direction.
to determine volume/moles of used up by the catalyst/sulfuric acid «in the titration»
OR
to eliminate/reduce «systematic» error caused by acid catalyst ✔
Do not accept “control” OR “standard” alone.
Percentage uncertainty:
✔
Percentage error:
✔
Award [1 max] if calculations are reversed OR if incorrect alcohol is used.
Any two:
large percentage error means large systematic error «in procedure» ✔
small percentage uncertainty means small random errors ✔
random errors smaller than systematic error ✔
Award [2] for “both random and systematic errors are significant.”
corrosive/burns/irritant/strong oxidizing agent/carcinogenic
OR
disposal is an environmental issue
OR
causes other side reactions/dehydration/decomposition ✔
Do not accept just “risk of accidents” OR “health risks” OR “hazardous”.
Examiners report
Well answered. Students mostly identified (alcohol) chain length as the independent variable and Kc at the dependent. For the latter [ethanoic acid] at equilibrium was another popular choice with some students neglecting to clarify "equilibrium" which was needed for the mark. This evidences an issue already identified in the Internal assessment that very often students only identify the processed variables. The proportion of students referring to volume of NaOH was too low for expectations.
A significant number of students scored at least one mark, usually the first and many both. Weaker students lost the second mark by referring to less collisions instead of less frequent collision or other words to this effect. Very few students referred to more diluted catalyst and of those even less were able to provide an adequate explanation in terms of the increased Ea. Many students tried to answer this question in terms of equilibrium instead of kinetics. There were also several responses that replied as if the dilution would only occur for part of the reaction or individual reactants instead of the entire solution.
Not well answered and of the few students that replied correctly most referred to preventing equilibrium shift and few candidates identified the direction of the shift. It was rather common to see answers where Le Chatelier's principle was stated without any attempt in adapting it to the context. Very few students described the specific equilibrium shift that could occur during the titration, changing the results.
Some students achieved on mark. Many answers referred simple to "control" or "standard" underlining the lack of some skills as also identified in the Internal assessment. Once again very few students had the specific details necessary to explain why this separate titration was needed in their response to receive a mark.
Many students scored both points and others at least one. Weaker students inverted the calculations.
Of the many students that obtained the mark most did through the first alternative and a lesser percentage through the third. Many students were unable to relate their calculations from 2e (percentage error and percentage uncertainty) to systematic error and random error. They either compared the calculations to incorrect errors or in some cases did not discuss the errors at all. Once again this points to a general lack on experimental understanding.
Most students received a mark for this question base on specific hazards. Very few students related disposal to environmental issues which isn't surprising as this is often missed in the Internal Assessment. Weaker students provided vague answers related to health issues which did not receive a mark. Some students misunderstood the question.
In a class experiment, students were asked to determine the value of x in the formula of a hydrated salt, BaCl2・xH2O. They followed these instructions:
- Measure the mass of an empty crucible and lid.
- Add approximately 2 g sample of hydrated barium chloride to the crucible and record the mass.
- Heat the crucible using a Bunsen burner for five minutes, holding the lid at an angle so gas can escape.
- After cooling, reweigh the crucible, lid and contents.
- Repeat steps 3 and 4.
Their results in three trials were as follows:
State and explain the further work students need to carry out in trial 2 before they can process the results alongside trial 1.
In trial 3, the students noticed that after heating, the crucible had turned black on the outside. Suggest what may have caused this, and how this might affect the calculated value for x in the hydrated salt.
List two assumptions made in this experiment.
Markscheme
repeat steps 3 and 4
OR
repeat step 5
OR
conduct a third heating
OR
«re»heat AND «re»weigh
water still present
OR
need two consistent readings
OR
heat to constant mass
Accept “ensure even/strong heating” for M1.
Do not accept “cleaning/washing the crucible”.
soot/carbon deposited
OR
incomplete combustion
OR
air hole of Bunsen burner closed/not fully open
Accept “using a yellow «Bunsen burner» flame” for M1.
«value of x» lower
Only award M2 if M1 correct.
all mass loss is due to water loss
all the water «of crystallization» is lost
crucible does not absorb/lose water
crystal/BaCl2 does not decompose/hydrolyse/oxidize/react with oxygen/air «when heated»
Accept “no loss of crystals/BaCl2 occurs”, “no impurities in the «weighed hydrated» salt”, “reaction goes to completion”, “heat was consistent/strong”, “crystal/BaCl2 does not absorb water during cooling”, “balance has been calibrated” or “crucible was clean at the start”.
Do not accept ”heat loss to surroundings” or “no carbon deposited on crucible”.
Reference to defects in apparatus not accepted.
Do not penalize if BaCl2.xH2O is used for BaCl2.
Examiners report
Students were asked to investigate how a change in concentration of hydrochloric acid, HCl, affects the initial rate of its reaction with marble chips, CaCO3.
They decided to measure how long the reaction took to complete when similar chips were added to 50.0 cm3 of 1.00 mol dm−3 acid and 50.0 cm3 of 2.00 mol dm−3 acid.
Two methods were proposed:
(1) using small chips, keeping the acid in excess, and recording the time taken for the solid to disappear
(2) using large chips, keeping the marble in excess, and recording the time taken for bubbles to stop forming.
A group recorded the following results with 1.00 mol dm−3 hydrochloric acid:
Annotate the balanced equation below with state symbols.
CaCO3(__) + 2HCl(__) → CaCl2(__) + CO2(__) + H2O(__)
Neither method actually gives the initial rate. Outline a method that would allow the initial rate to be determined.
Deduce, giving a reason, which of the two methods would be least affected by the chips not having exactly the same mass when used with the different concentrations of acid.
State a factor, that has a significant effect on reaction rate, which could vary between marble chips of exactly the same mass.
Justify why it is inappropriate to record the uncertainty of the mean as ±0.01 s.
If doubling the concentration doubles the reaction rate, suggest the mean time you would expect for the reaction with 2.00 mol dm−3 hydrochloric acid.
Another student, working alone, always dropped the marble chips into the acid and then picked up the stopwatch to start it. State, giving a reason, whether this introduced a random or systematic error.
Markscheme
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Accept “CO2(aq)”.
[1 mark]
measure the volume of gas at different times «plot a graph and extrapolate»
OR
measure the mass of the reaction mixture at different times «plot a graph and extrapolate»
Accept other techniques that yield data which can be plotted and extrapolated.
[1 mark]
method 2 AND marble is in excess «so a little extra has little effect»
OR
large chips AND marble is in excess «so a little extra has little effect»
OR
method 2 AND HCl is limiting reagent «so a little extra marble has little effect»
OR
large chips AND HCl is limiting reagent «so a little extra marble has little effect»
Accept, as a reason, that “as the mass is greater the percentage variation will be lower”.
[1 mark]
surface area
OR
purity «of the marble»
Accept “shape of the chip”.
[1 mark]
variation of individual values is much greater «than this uncertainty»
OR
«uncertainty» does not take into account «student» reaction time
[1 mark]
« = 60.98 s» = 61 «s»
[1 mark]
systematic AND always makes the time shorter «than the actual value»
OR
systematic AND it is an error in the method used «not an individual measurement»
OR
systematic AND more repetitions would not reduce the error
Accept, as reason, “it always affects the value in the same direction” OR “the error is consistent”.
[1 mark]
Examiners report
Body fluids have different pH values.
Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
Outline how ranitidine reduces stomach acidity.
Calculate the pH of a buffer solution which contains 0.20 mol dm−3 ethanoic acid and 0.50 mol dm−3 sodium ethanoate. Use section 1 of the data booklet.
pKa (ethanoic acid) = 4.76
Markscheme
hydrochloric acid/HCl «(aq)» AND strong «acid» ✔
MgCO3 (s) + 2HCl (aq) → MgCl2 (aq) + CO2 (g) + H2O (l) ✔
NOTE: Accept ionic equation.
blocks/binds to H2-histamine receptors «in cells of stomach lining»
OR
prevents histamine molecules binding to H2-histamine receptors «and triggering acid secretion»
OR
prevents parietal cells from releasing/producing acid ✔
NOTE: Do not accept “antihistamine” by itself.
Accept “H2-receptor antagonist/H2RA” OR “blocks/inhibits action of histamine”.
Accept “blocks receptors in parietal cells «from releasing/producing acid»”.
Do not accept “proton pump/ATPase inhibitor”.
«pKa = 4.76»
«pH = pKa + log »
«pH = 4.76 + 0.40 =» 5.16 ✔
Examiners report
Ethanol was electrolysed at different voltages. The products at the anode, ethanoic acid, ethanal and carbon dioxide, were collected and analysed.
The percentages of products obtained using three different catalysts mounted on a carbon anode, platinum (Pt/C), platinum and ruthenium alloy (PtRu/C) and platinum and tin alloy (PtSn/C) are shown.
Chemical yields of ethanoic acid, ethanal and carbon dioxide as a function of voltage for
oxidation of 0.100 mol dm−3 ethanol at Pt/C, PtRu/C and PtSn/C anodes at 80°C.
[Source: Product Distributions and Efficiencies for Ethanol Oxidation in a Proton Exchange Membrane Electrolysis Cell, Rakan M. Altarawneh and Peter G. Pickup, Journal of the Electrochemical Society, 2017, volume 164, issue 7, http://jes.ecsdl.org/. Distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/)]
Describe the effect of increasing the voltage on the chemical yield of:
Ethanal using Pt/C:
Carbon dioxide using PtRu/C:
Determine the change in the average oxidation state of carbon.
From ethanol to ethanal:
From ethanol to carbon dioxide:
List the three products at the anode from the least to the most oxidized.
Deduce, giving your reason, which catalyst is most effective at fully oxidizing ethanol.
Markscheme
Ethanal using Pt/C:
decreases ✔
Carbon dioxide using PtRu/C:
«generally» increases AND then decreases ✔
NOTE: Accept “no clear trend/pattern” OR “increases and decreases” OR “increases, reaches a plateau and «then» decreases” for M2.
From ethanol to ethanal:
−2 to −1
OR
+1/increases by 1 ✔
NOTE: Do not accept “2− to 1−”.
From ethanol to carbon dioxide:
−2 to +4
OR
+6/increases by 6 ✔
NOTE: Do not accept “2− to 4+”.
Do not penalize incorrect notation twice.
Penalize incorrect oxidation state value of carbon in ethanol once only.
ethanal < ethanoic acid < carbon dioxide ✔
NOTE: Accept formulas.
No ECF from 2aii calculations.
Pt/platinum/PtC AND highest yield of CO2 «at all voltages» ✔
NOTE: ECF from 2aiii.
Examiners report
Palmitic acid has a molar mass of 256.5 g mol−1.
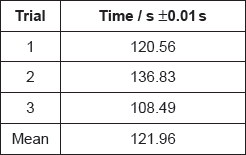
The apparatus in the diagram measures the surface pressure created by palmitic acid molecules on the surface of water. This pressure is caused by palmitic acid molecules colliding with the fixed barrier. The pressure increases as the area, A, available to the palmitic acid is reduced by the movable barrier.
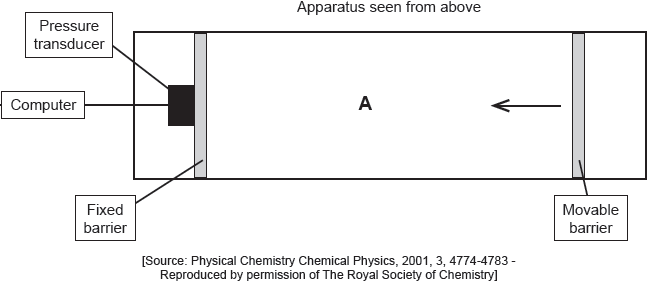
When a drop of a solution of palmitic acid in a volatile solvent is placed between the barriers, the solvent evaporates leaving a surface layer. The graph of pressure against area was obtained as the area A was reduced.
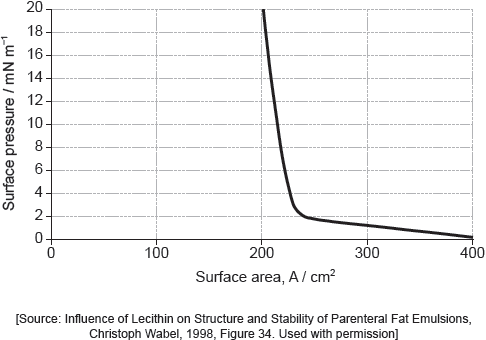
Part of this molecule is hydrophilic (bonds readily to water) and part hydrophobic (does not bond readily to water). Draw a circle around all of the hydrophilic part of the molecule.
When a small amount of palmitic acid is placed in water it disperses to form a layer on the surface that is only one molecule thick. Explain, in terms of intermolecular forces, why this occurs.
Suggest why there is a small increase in the surface pressure as the area is reduced to about 240 cm2, but a much faster increase when it is further reduced.
The solution of palmitic acid had a concentration of 0.0034 mol dm−3. Calculate the number of molecules of palmitic acid present in the 0.050 cm3 drop, using section 2 of the data booklet.
Assuming the sudden change in gradient occurs at 240 cm2, calculate the area, in cm2, that a single molecule of palmitic acid occupies on surface of the water.
If you did not obtain an answer for (b)(ii) use a value of 8.2 × 1016, but this is not the correct answer.
Markscheme
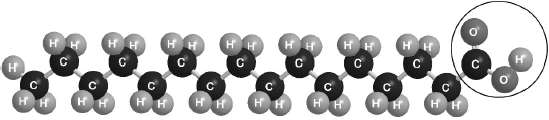
Must cut CH2–CO bond AND enclose all of the –COOH group.
[1 mark]
Any two of:
–COOH/CO/OH/carboxylate/carboxyl/hydroxyl/hydroxy group forms hydrogen bonds/H-bonds to water
London/dispersion/instantaneous induced dipole-induced dipole forces occur between hydrocarbon chains
hydrocarbon chain cannot form hydrogen bonds/H-bonds to water
strong hydrogen bonds/H-bonds between water molecules exclude hydrocarbon chains «from the body of the water»
Accept “hydrophilic part/group forms hydrogen bonds/H-bonds to water”.
Accept “hydrophobic section” instead of “hydrocarbon chain”.
Award [1 max] for answers based on “the –COOH group being polar AND the hydrocarbon chain being non-polar”.
[2 marks]
Above about 240 cm2:
greater collision frequency/collisions per second between «palmitic acid» molecules and the barrier «as area reduced»
At less than about 240 cm2:
molecules completely cover the surface
OR
there is no space between molecules
OR
force from movable barrier transmitted directly through the molecules to the fixed barrier
OR
«palmitic acid» molecules are pushed up/down/out of layer
For both M1 and M2 accept “particles” for “molecules”.
For M1 accept “space/area between molecules reduced” OR “molecules moving closer together”.
[2 marks]
amount of acid = «5.0 × 10–5 dm3 × 0.0034 mol dm–3» = 1.7 × 10–7 «mol»
number of molecules = «1.7 × 10–7 mol × 6.02 × 1023 mol–1 =» 1.0 × 1017
Award [2] for correct final answer.
Award [1] for “1.0 × 1020”.
[2 marks]
«area = » 2.4 × 10–15 «cm2»
[1 mark]
Examiners report
A class was determining the concentration of aqueous sodium hydroxide by titrating it with hydrochloric acid, whilst monitoring the pH of the solution. The sodium hydroxide solution was added into a glass beaker from a measuring cylinder and the hydrochloric acid added using a burette. One group of students accidentally used a temperature probe rather than a pH probe. Their results are given below.
Volume of aqueous NaOH = 25.0 ± 0.5 cm3
Concentration of HCl = 1.00 ± 0.01 mol dm−3
State and explain how the graph would differ if 1 moldm−3 sulfuric acid had been used instead of 1 moldm−3 hydrochloric acid.
Markscheme
graph would peak/maximum at 17.5 cm3
OR
smaller volume of acid «needed to reach equivalence»
sulfuric acid is dibasic/diprotic
higher temperature would be reached
Accept “gradient/slope «of graph» is greater/steeper” for M1.
Accept “one mole of sulfuric acid neutralizes two moles of NaOH” for M2.
[2 marks]
Examiners report
Alloys containing at least 60 % copper reduce the presence of bacteria on their surface.The percentage of copper in brass, an alloy of copper and zinc, can be determined by UV-vis spectrometry.
A sample of brass is dissolved in concentrated nitric acid and then made up to 250.0 cm3 with water before analysis.
Cu (s) + 4HNO3 (aq) → Cu(NO3)2 (aq) + 2NO2 (g) + 2H2O (l)
3Zn (s) + 8HNO3 (aq) → 3Zn(NO3)2 (aq) + 2NO (g) + 4H2O (l)
The concentration of copper(II) ions in the resulting solution is then determined from a calibration curve, which is plotted by measuring the light absorbance of standard solutions.
Titration is another method for analysing the solution obtained from adding brass to nitric acid.
Outline why the initial reaction should be carried out under a fume hood.
Deduce the equation for the relationship between absorbance and concentration.
Outline how a solution of 0.0100 mol dm−3 is obtained from a standard 1.000 mol dm−3 copper(II) sulfate solution, including two essential pieces of glassware you would need.
The original piece of brass weighed 0.200 g. The absorbance was 0.32.
Calculate, showing your working, the percentage of copper by mass in the brass.
Deduce the appropriate number of significant figures for your answer in (d)(i).
Comment on the suitability of using brass of this composition for door handles in hospitals.
If you did not obtain an answer to (d)(i), use 70 % but this is not the correct answer.
Suggest another property of brass that makes it suitable for door handles.
Copper(II) ions are reduced to copper(I) iodide by the addition of potassium iodide solution, releasing iodine that can be titrated with sodium thiosulfate solution, Na2S2O3 (aq). Copper(I) iodide is a white solid.
4I− (aq) + 2Cu2+ (aq) → 2CuI (s) + I2 (aq)
I2 (aq) + 2S2O32− (aq) → 2I− (aq) + S4O62− (aq)
Deduce the overall equation for the two reactions by combining the two equations.
Suggest why the end point of the titration is difficult to determine, even with the addition of starch to turn the remaining free iodine black.
Markscheme
NO2/NO/NOx/HNO3/gas is poisonous/toxic/irritant ✔
Accept formula or name.
Accept “HNO3 is corrosive” OR “poisonous/toxic gases produced”.
Accept “reaction is harmful/hazardous”.
Slope (gradient):
40 ✔
Equation:
absorbance = 40 × concentration
OR
y = 40x ✔
Accept any correct relationship for slope such as .
Award [2] if equation in M2 is correct.
dilute 1.00 cm3 «of the standard solution with water» to 100 cm3
OR
dilute sample of standard solution «with water» 100 times ✔
«graduated/volumetric» pipette/pipet ✔
volumetric flask ✔
Accept any 1 : 100 ratio for M1.
Accept “mix 1 cm3 of the standard solution with 99 cm3 of water” for M1.
Do not accept “add 100 cm3 of water to 1.00 cm3 of standard solution” for M1.
Accept “burette/buret” for M2.
Accept “graduated/measuring flask” for M3 but not “graduated/measuring cylinder” or “conical/Erlenmeyer flask”.
concentration of copper = 0.0080 «mol dm–3» ✔
mass of copper in 250.0 cm3 = «0.0080 mol dm–3 × 0.2500 dm3 × 63.55 g mol–1 =» 0.127 «g»
OR
mass of brass in 1 dm3 = «4 × 0.200 g =» 0.800 g AND [Cu2+] = «0.0080 mol dm–3 × 63.55 g mol–1 =» 0.5084 g dm–3 ✔
«% copper in this sample of brass » 64 «%»
OR
«% copper in this sample of brass » 64 «%» ✔
Accept any value in range 0.0075–0.0085 «mol dm–3» for M1.
Accept annotation on graph for M1.
Award [3] for correct final answer.
Accept “65 «%»”.
two ✔
Do not apply ECF from 1(d)(i).
«since it is greater than 60%» it will reduce the presence of bacteria «on door handles» ✔
resistant to corrosion/oxidation/rusting
OR
low friction surface «so ideal for connected moving components» ✔
Accept “hard/durable”, “«high tensile» strength”, “unreactive”, “malleable” or any reference to the appearance/colour of brass (eg “gold-like”, “looks nice” etc.).
Do not accept irrelevant properties, such as “high melting/boiling point”, “non-magnetic”, “good heat/electrical conductor”, “low volatility”, etc.
Do not accept “ductile”.
2I− (aq) + 2Cu2+ (aq) + 2S2O32− (aq) → 2CuI (s) + S4O62− (aq)
correct reactants and products ✔
balanced equation ✔
M2 can only be awarded if M1 is correct.
precipitate/copper(I) iodide/CuI makes colour change difficult to see
OR
release of I2/iodine from starch-I2 complex is slow so titration must be done slowly ✔
Examiners report
Consider the following lipid and carbohydrate.
In order to determine the number of carbon-carbon double bonds in a molecule of linoleic acid, 1.24 g of the lipid were dissolved in 10.0 cm3 of non-polar solvent.
The solution was titrated with a 0.300 mol dm–3 solution of iodine, I2.
Determine the empirical formula of linoleic acid.
The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per gram than fructose.
State the type of reaction occurring during the titration.
Calculate the volume of iodine solution used to reach the end-point.
Outline the importance of linoleic acid for human health.
Markscheme
C9H16O
ratio of oxygen to carbon in linoleic acid lower
OR
linoleic acid less oxidized
OR
linoleic acid more reduced
Accept “«average» oxidation state of carbon in linoleic acid is lower”.
«electrophilic» addition/AE
OR
oxidation–reduction/redox
« =» 0.00442 «mol»
0.00884 mol of C=C
OR
ratio of linoleic acid : iodine = 1:2
«volume of I2 solution = =» 0.0295 «dm3» / 29.5 «cm3»
Award [3] for correct final answer.
Any two of:
increases «ratio of» HDL «to LDL» cholesterol
OR
decreases LDL cholesterol «level»
removes plaque from/unblocks arteries
OR
decreases risk of heart disease
decreases risk of stroke «in the brain»
Accept "essential fatty acid".
Do not accept “bad cholesterol” for “LDL cholesterol” OR “good cholesterol” for “HDL cholesterol”.
Do not accept general answers such as “source of energy” OR “forms triglycerides” OR “regulates permeability of cell membranes” etc.
[Max 2 Marks]
Examiners report
Physical properties of elements vary according to atomic number. Sections 6 to 9 of the data booklet list some of these properties.
Deduce, giving a reason, the group of elements in the periodic table most likely to undergo sublimation.
Describe the density trend across periods 4 and 5 of the periodic table.
Suggest, with a reason, whether the lanthanoids or actinoids of the f-block would have the higher density.
Compare the ease of oxidation of s-block and d-block metals to their melting points and densities. Use section 25 of the data booklet.
Sketch how the first ionization energies of elements vary with their atomic radius.
Markscheme
group 18/noble gases [✔]
smallest difference between melting and boiling points
OR
weakest intermolecular forces «in that period» [✔]
Note: Accept “group 17/halogens”.
density increases «to a maximum in the transition elements» AND then decreases [✔]
actinoids AND density increases down all groups «due to large increase in atomic mass for small increase in atomic volume»
OR
actinoids AND «much» greater atomic mass with similar type of bonding
OR
actinoids AND density «of actinoids» atomic number 90 to 95 is greater than corresponding lanthanoids [✔]
Note: Accept “actinoids AND on graph actinoids have «much» greater density than lanthanoids”.
Alternative 1:
«metals with» low densities oxidize easier [✔]
«metals with» low melting points oxidize easier [✔]
Alternative 2:
in s-block «metals with» high densities oxidize easier
OR
in s-block «metals with» low melting points oxidize easier [✔]
in d-block «metals with» low densities oxidize easier
OR
in d-block «metals with» low melting points oxidize easier [✔]
Note: Award [1 max] for “s-block metals more easily oxidized” OR “s-block metals have lower melting points” OR “s-block metals have lower densities”.
Accept “have greater activity” for “oxidize easier”.
[✔]
Note: Accept any negative sloping line.
Do not award mark if line touches either axis.
Examiners report
Some candidates appeared to be unfamiliar with the term “sublimation”. Whilst most students correctly identified the noble gases as the group most likely to sublime there were a variety of other answers. Many students referred to “low melting and boiling points” rather than identifying the difference between these as the key factor.
Most students realised that density peaks around the middle of each of these periods, though a significant minority seemed unaware of the significance of “periods” and just reported the overall general increase in density.
Poorly answered. Some students seemed unaware of the terms “lanthanoids” and “actinoids”. Many others just stated the actinoids because they had greater atomic masses, without adding that the bonding, and hence the volume occupied by each atom, would be similar to the lanthanoids. Others responded in terms of the given data, but this required rather more justification than just stating “as can be seen from the graph”.
Almost all candidates gained some credit on this question and many obtained full marks. Students were generally aware that s-block elements are more reactive than d-block ones and hence are more easily oxidised. Many correctly linked this to lower melting points and densities. Often a causal relationship was implied (more reactive because of their low density/mp) but this was not penalised. A significant minority of students referred to only one of the physical properties; not reading the question fully?
Most students sketched a negative correlation between IE and radius, but then many lost the mark by drawing a line that met an axis; it is not possible for either to have a value of zero.
Sodium chloride, NaCl, can be spread on icy roads to lower the freezing point of water.
The diagram shows the effects of temperature and percentage by mass of NaCl on the composition of a mixture of NaCl and H2O.
Estimate the lowest freezing point of water that can be reached by adding sodium chloride.
Estimate the percentage by mass of NaCl dissolved in a saturated sodium chloride solution at +10 ºC.
Calculate the percentage of water by mass in the NaCl•2H2O crystals. Use the data from section 6 of the data booklet and give your answer to two decimal places.
Suggest a concern about spreading sodium chloride on roads.
Markscheme
–21 «ºC»
28 «%»
Accept any specific answer in the range 27 to 29 «%».
Mr = 94.48
«» 38.15 «%»
Award M2 only if answer is to 2 decimal places.
Award [2] for correct final answer.
Award [1 max] for 38.10 %.
rust/corrosion «of cars and bridges»
OR
waste of important raw material
OR
soil/water salination/pollution «from run off»
OR
erosion of/damage to the road surface
OR
specific example of damage to the ecosystem
OR
«outdoor» temperatures may go below effective levels for NaCl «to lower freezing point» so NaCl could be wasted
OR
roads can refreeze causing hazards
Do not accept “tyre damage”.
Do not accept “economic issues” OR “environmental issues” unless specified (eg accept “increase in costs for local councils road budgets” but not “cost” alone).
Do not accept “makes roads more slippery”.
Examiners report
Describe the characteristics of the nematic liquid crystal phase and the effect that an electric field has on it.
Shape of molecules:
Distribution:
Effect of electric field:
Markscheme
Shape of molecules:
linear
OR
rod «shaped» [✔]
Distribution:
no positional order AND «some» directional order [✔]
Note: Accept “partly ordered”.
Effect of electric field:
«directional» order increases
OR
molecules align in same direction [✔]
Examiners report
Most students were able to obtain at least one mark on this question. The distribution was the most challenging part.
Consider the following antacids:
Show that antacid X is more effective, per tablet, than antacid Y.
Markscheme
same reactant mole ratio «in both equations»
OR
AND
✔
AND
✔
«tablet of» X neutralizes AND «tablet of» Y neutralizes ✔
Award [3] for correct final statement AND values in M3.
Examiners report
Very poorly answered. Many students scored at least 1 point. This was usually M2 by determining the mols
of each antacid present. However, many students totally ignored the corresponding balanced chemical equations or entered wrong ones. A worrying number of candidates didn't therefore use the correct molar ratio to calculate the values in M3. Showing the calculations and how X is more effective than Y was the point of the question.
Disposable plastic lighters contain butane gas. In order to determine the molar mass of butane, the gas can be collected over water as illustrated below:
List the data the student would need to collect in this experiment.
Explain why this experiment might give a low result for the molar mass of butane.
Suggest one improvement to the investigation.
Markscheme
mass/m of lighter before AND after the experiment
volume of gas/Vgas «collected in the cylinder»
«ambient» pressure/P «of the room»
temperature/T
Accept “change in mass of lighter”.
Accept “weight” for “mass”.
Do not accept just “mass of lighter/gas”.
Accept “volume of water displaced”.
Do not accept “amount” for “volume” or “mass”.
[4 marks]
Any two of:
pressure of gas not equalized with atmospheric/room pressure
too large a recorded volume «of gas produces a lower value for molar mass of butane»
OR
cylinder tilted
difficult to dry lighter «after experiment»
OR
higher mass of lighter due to moisture
OR
smaller change in mass but same volume «produces lower value for molar mass of butane»
using degrees Celcius/°C instead of Kelvin/K for temperature
Accept “vapour pressure of water not accounted for” OR “incorrect vapour pressure of water used” OR “air bubbles trapped in cylinder”. Do not accept “gas/bubbles escaping «the cylinder»” or other results leading to a larger molar mass.
Accept “lighter might contain mixture of propane and butane”.
Do not accept only “human errors” OR “faulty equipment” (without a clear explanation given for each) or “mistakes in calculations”.
[2 marks]
record vapour pressure of water «at that temperature»
OR
equalize pressure of gas in cylinder with atmospheric/room pressure
OR
tap cylinder before experiment «to dislodge trapped air»
OR
collect gas using a «gas» syringe/eudiometer/narrower/more precise graduated tube
OR
collect gas through tubing «so lighter does not get wet»
OR
dry lighter «before and after experiment»
OR
hold «measuring» cylinder vertical
OR
commence experiment with cylinder filled with water
Accept “adjust cylinder «up or down» to ensure water level inside cylinder matches level outside”.
Accept “repeat experiment/readings «to eliminate random errors»”.
Accept “use pure butane gas”.
[1 mark]
Examiners report
Aspirin is one of the most widely used drugs in the world.
Aspirin was synthesized from 2.65 g of salicylic acid (2-hydroxybenzoic acid) (Mr = 138.13) and 2.51 g of ethanoic anhydride (Mr = 102.10).
Calculate the amounts, in mol, of each reactant.
Calculate, in g, the theoretical yield of aspirin.
State two techniques which could be used to confirm the identity of aspirin.
State how aspirin can be converted to water-soluble aspirin.
Compare, giving a reason, the bioavailability of soluble aspirin with aspirin.
Markscheme
n(salicylic acid) = «» 0.0192 «mol»
AND
n(ethanoic anhydride) = «» 0.0246 «mol»
[1 mark]
«mass = 0.0192 mol x 180.17 gmol–1 =» 3.46 «g»
Award ECF mark only if limiting reagent determined in (i) has been used.
[1 mark]
Any two of:
melting point
mass spectrometry/MS
high-performance liquid chromatography/HPLC
NMR/nuclear magnetic resonance
X-ray crystallography
elemental analysis «for elemental percent composition»
Accept “spectroscopy” instead of “spectrometry” where mentioned but not “spectrum”.
Accept “infra-red spectroscopy/IR” OR “ultraviolet «-visible» spectroscopy/UV/UV-Vis”.
Do not accept “gas chromatography/GC”.
Accept “thin-layer chromatography/TLC” as an alternative to “HPLC”.
[2 marks]
react with NaOH
Accept “NaHCO3” or “Na2CO3” instead of “NaOH”.
Accept chemical equation OR name for reagent used.
[1 mark]
«marginally» higher AND increase rate of dispersion
OR
«marginally» higher AND increase absorption in mouth/stomach «mucosa»
OR
«approximately the» same AND ionic salt reacts with HCl/acid in stomach to produce aspirin again
Do not accept “«marginally» higher AND greater solubility in blood”.
[1 mark]
Examiners report
Gasoline (petrol), biodiesel and ethanol are fuels.
[U.S. Department of Energy. https://afdc.energy.gov/]
Calculate the energy released, in , from the complete combustion of of ethanol.
State a class of organic compounds found in gasoline.
Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.
A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture over the use of pure gasoline. Exclude any discussion of cost.
Contrast the molecular structures of biodiesel and the vegetable oil from which it is formed.
When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
Suggest a wavenumber absorbed by methane gas.
Markscheme
✔
alkane
OR
cycloalkane
OR
arene ✔
Accept “alkene”.
Do not accept just “hydrocarbon”, since given in stem.
Do not accept “benzene/aromatic” for “arene”.
Advantages: [2 max]
renewable ✔
uses up waste «such as used cooking oil» ✔
lower carbon footprint/carbon neutral ✔
higher flashpoint ✔
produces less /
OR
less polluting emissions ✔
has lubricating properties
OR
preserves/increases lifespan of engine ✔
increases the life of the catalytic converter ✔
eliminates dependence on foreign suppliers ✔
does not require pipelines/infrastructure «to produce» ✔
relatively less destruction of habitat compared to obtaining petrochemicals ✔
Accept “higher energy density” OR “biodegradable” for advantage.
Disadvantages: [2 max]
needs conversion/transesterification ✔
takes time to produce/grow plants ✔
takes up land
OR
deforestation ✔
fertilizers/pesticides/phosphates/nitrates «used in production of crops» have negative environmental effects ✔
biodiversity affected
OR
loss of habitats «due to energy crop plantations» ✔
cannot be used at low temperatures ✔
variable quality «in production» ✔
high viscosity/can clog/damage engines ✔
Accept “lower specific energy” as disadvantage.
Do not accept “lower octane number” as disadvantage”.
Any one:
uses up fossil fuels more slowly ✔
lower carbon footprint/CO2 emissions ✔
undergoes more complete combustion ✔
produces fewer particulates ✔
higher octane number/rating
OR
less knocking ✔
prevents fuel injection system build up
OR
helps keep engine clean ✔
Accept an example of a suitable advantage even if repeated from 9c.
Any two:
biodiesel has smaller molecules/single «hydrocarbon» chain AND oil has larger molecules/multiple «hydrocarbon» chains ✔
biodiesel is methyl/ethyl ester AND oil has «backbone of» glycerol joined to fatty acids ✔
biodiesel contains one ester group AND oil contains three ester groups ✔
Do not accept properties such as “less viscous” or “lower ignition point”.
carbon dioxide allows sunlight/short wavelength radiation to pass through AND particulates reflect/scatter/absorb sunlight ✔
Accept “particulates reflect/scatter/absorb sunlight AND carbon dioxide does not”.
Accept “ absorbs «radiation» AND particulates reflect/scatter/absorb sunlight”.
Do not accept “traps” for “absorbs”.
carbon dioxide is highly/more abundant «in the atmosphere» ✔
methane is more effective/potent «as a greenhouse gas»
OR
methane/better/more effective at absorbing «radiation»
OR
methane has greater greenhouse factor
OR
methane has greater global warming potential/GWP✔
Accept “carbon dioxide contributes more to global warming” for M1.
any value or range within ✔
Examiners report
Even rather weak candidates answered this one correctly.
Most candidates answered alkanes with a lower number stating hydrocarbons or benzene and therefore lost the mark.
There were many good answers, but few candidates fully scored. Higher energy density and lower specific energy were quite common, and so references to damaging engines. Many students spent more time explaining each advantage rather than simply outlining. There were fewer journalistic and generic answers for this type of question than in the past.
Another question where many candidates obtained the mark. In quite a few cases students repeated the argument for (c) and this allowed them to get two points for the same answer.
Quite disappointing with few candidates producing answers that showed deep understanding. Answers such as less viscous or lower ignition point were common. This question specifically asks for contrasts in the structures not the properties of the compounds. Students need to be reminded that a contrast statement requires something about each substance.
Showed a wide variety of answers but is was worrying that many students limited to explain the greenhouse effect. There were many responses that did not answer the question or only gave a response for one of the 2 substances.
We received many good answers, but it was worrying the number of students that still provided general and shallow comments. Of the 3 contrast question this had the best response.
Many good answers with some students losing the mark as didn't read or understand the question correctly and provided answers in terms of wavelengths.
A class was determining the concentration of aqueous sodium hydroxide by titrating it with hydrochloric acid, whilst monitoring the pH of the solution. The sodium hydroxide solution was added into a glass beaker from a measuring cylinder and the hydrochloric acid added using a burette. One group of students accidentally used a temperature probe rather than a pH probe. Their results are given below.
Volume of aqueous NaOH = 25.0 ± 0.5 cm3
Concentration of HCl = 1.00 ± 0.01 mol dm−3
Suggest how the end point of the titration might be estimated from the graph.
Markscheme
volume «found by extrapolation of the two best fit lines» required to give the highest temperature
OR
extrapolate «two best fit» lines to the point where they meet
Accept “where lines through the points meet”.
Accept “at maximum temperature”.
Accept “at 35 cm3 of HCl”.
[1 mark]
Examiners report
This question is about a mug made of a lead alloy.
The rate of lead dissolving in common beverages with various pH values was analysed.
Identify the experiment with the highest rate of lead dissolving.
Suggest why the relationship between time and lead concentration for Cola at 16 °C is not linear.
Lead(II) chloride, PbCl2, has very low solubility in water.
PbCl2 (s) Pb2+ (aq) + 2Cl− (aq)
Explain why the presence of chloride ions in beverages affects lead concentrations.
A mean daily lead intake of greater than 5.0 × 10−6 g per kg of body weight results in increased lead levels in the body.
Calculate the volume, in dm3, of tap water from experiment 8 which would exceed this daily lead intake for an 80.0 kg man.
Markscheme
6 [✔]
Note: Accept “orange juice”.
equilibrium is being established «between lead in solution and in mug»
OR
solution becoming saturated
OR
concentration of lead ions/[Pb2+] has increased «over time»
OR
acid concentration has decreased «as reacted with lead»
OR
surface lead has decrease/formed a compound/forms insoluble layer on surface
OR
acid reacts with other metals «because it is an alloy» [✔]
Note: Do not accept “concentration of cola, orange juice, etc… has decreased”.
Do not accept responses that only discusses mathematical or proportional relationships.
equilibrium shifts to the left/towards reactants [✔]
lead «compounds/ions» precipitate
OR
concentration of lead «ions»/[Pb2+] decreases [✔]
Note: Award [2] for “equilibrium shifts to the left/towards reactants due to common ion effect”.
Accept “lead ions/[Pb2+] removed from solution” for M2.
«daily limit = 5.0 × 10–6 g kg–1 × 80.0kg =» 4.0 × 10–4 «g of lead» [✔]
«volume =» 2.7 × 10–2/0.027 «dm3» [✔]
Note: Award [2] for correct final answer.
Examiners report
Most candidates did well on this question, identifying the correct experiment by number or beverage.
Many candidates struggled with this question, answering it from a mathematical perspective rather than explaining why the rate would decrease over time from a chemical perspective. There were several possible correct answers (reaching equilibrium, acid concentration decreasing, solution becoming saturated with lead ions, etc.…)
This question was an equilibrium question. Many students received 1 mark for either concentration of lead decreased, or lead chloride was produced and quite a few recognized that the explanation was the reaction shifted to the reactant or left side for the second mark.
Most students receive one mark for this question, and many receive both marks. The most common mistakes involved incorrect conversions from gram to milligrams or milligrams to grams.